
Christine King BVSc, MANZCVS (equine), MVetClinStud
Through the Looking-Glass
a bug's-eye view of the equine gut
and what it can tell us about feeding horses
© Christine M. King
last revised 14Jan2020
Chapter 1
"What's one and one and one and one and one
and one and one and one and one and one?"
"I don't know," said Alice. "I lost count."
Bacterial Diversity
The diversity, or number of different types of bacteria, in the equine gut is perhaps even more mind-boggling, particularly when one reads the labels on probiotic products for horses, many of which contain, at most, only three or four different bacterial species or strains. (More on probiotics in Chapter 3.)
First, a bit of background on the methods used to study microbial diversity, or 'richness'. As with estimates of total bacterial numbers, early studies on the diversity of the gut microbiota used bacterial culture. These methods are very good for identifying and characterising the gut bacteria that will grow in culture, but they miss many, and evidently most, of the bacterial species that live in the equine gut.
There are now several different culture-independent methods that are used to identify and classify the gut microbiota. Each has its place in examining this incredibly complex ecosystem; but each also has its limitations.
For examining the diversity of the equine gut microbiota, casting the net as wide as possible, the most commonly used methods look for specific gene sequences (mostly in the V1–V8 regions of the 16S ribosomal RNA gene, for us science nerds). Typically, bacteria that share at least 97% similarity in the target gene sequence are grouped together and called an operational taxonomic unit (OTU).
Although there are some exceptions to the rule, and the reference databases are constantly being updated, an OTU at this level of similarity (97%) is roughly equivalent to a single species or an otherwise very small group of closely-related bacteria. So for our purposes, we’ll assume that the number of different OTUs in a sample of faeces or gut contents represents the number of different bacterial species in the sample (unless a lower similarity threshold is used).
Number of bacterial species
Using these techniques, the number of different OTUs found in the faeces of healthy horses averages between 1,500 and 2,000 per horse, and in some studies and horses it's well over 3,000 per horse. Furthermore, for practical reasons it's common to end the OTU count before a plateau is reached, so the true count is probably higher still.
Statistical estimates of the number of different bacterial species, including those found with very low frequency or even just once, in the faeces of healthy horses, average from 2,000 to more than 10,000 per horse.
- One of these referenced studies involved donkeys.They're included here as their bacterial diversity and profiles were similar to those of horses.
While these statistically derived estimates may prove to be overestimates (at least, practically speaking), the fact remains that, on average...
there are more than 1,500 different bacterial species in the faeces,
and therefore in the hindgut, of healthy horses.
Even in the stomach, a gut compartment not known for its microbial contribution to digestion, healthy horses may harbour more than 130 different bacterial species. (More on that at the end of the chapter.)
Think about all that for a moment: off the top of your head, can you name even ten different species of bacteria that might be found in the gut?
For most of us, our perception of the gut microbes is limited to just a handful of organisms, mostly common pathogens such as Escherichia coli (E. coli), Clostridium difficile (‘C. diff’), and Salmonella, or names on the labels of probiotic products, such as Lactobacillus acidophilus and Bifidobacterium something.
The more we learn about the tremendous diversity of the equine gut microbiota, the more it challenges our perception of the role it plays in health and disease, and how—or even whether—we can work with this microcosm without inadvertently creating new problems for the horse.
I find it troubling that the main driver of current research on the gut microbiota in animals is the prospect of 'exploiting' the microbiota for our own ends. Those ends range from better performance in livestock or sporthorses kept in unnatural conditions and fed unnatural diets, to the search for novel antibiotics and other drugs. We just can't leave well enough alone!
The 'core' or common microbiome
Even more challenging to our habitual way of proceeding—of aiming to know a thing by identifying all its parts and pinning them down with labels, like dead butterflies to a cork board—is this: very few of the 1,500-plus species of bacteria found in the faeces or hindgut of healthy horses are found in all, or even in most, of the horses in a study, even when the horses are on the same farm, fed the same diet, and sampled at the same time of year.
For example, in a group of eight Thoroughbred geldings (castrated male horses), all in training at the same racing stable and all fed the same diet, the number of different OTUs (bacterial species) in the faeces ranged from 1,200 to 3,000 per horse, but only 67 OTUs were found in all eight horses. In other words, less than 6% of the OTUs identified in this group of horses were common to all.
Another study set out with the primary goal of characterising the ‘core faecal bacterial microbiome’ of Irish Thoroughbred racehorses, independent of diet, management, geographic location, or age. (Spoiler Alert: Turns out that diet, management, geographic location, and even social interactions have a great deal to do with the gut microbiota.)
In the six horses studied*, the number of different OTUs in the faeces ranged from 1,755 to 2,736 per horse, but only 13 identifiable species were found in at least four of the six horses (the study's definition of 'core'), and only 7 identifiable species were found in all six horses.
- * This problem, of reaching conclusions based on only a handful of horses, comes up again and again with studies of the equine gut microbiota. Just as “One swallow does not a summer make...,” studying too few individuals can lead to conclusions that, at best, are incomplete, and at worst are incorrect.
One floor up, at the Genus level, the researchers had better luck: 34 identifiable genera (genus, plural) were found in at least four of the six horses, and 24 identifiable genera were found in all six horses.
However, the ‘identifiable’ descriptor is a rather important fly-in-the-ointment of this effort to characterise the 'core' microbiota of these horses, because more than 50% of the OTUs could not be identified and thus classified even at the genus level. In three of the six horses, only about 40% of OTUs were identifiable at the genus level.
For a bit of everyday context, a genus-level analysis of animals would pin-down dogs to the genus Canis, but it would not distinguish between dogs, coyotes, wolves, jackals, dingoes, African wild dogs, and others in this genus. So while we'd have a fairly cohesive collection of mid-sized, dog-like carnivores, we would be missing a good deal about the habits and habitats of the individual species within this genus.
The differences among species in the same genus of bacteria may not be as dramatic, but if there were not identifiable and important enough differences between bacterial species, then there would be no need for separate species identifiers. One cannot help but see the importance of distinguishing Clostridium botulinum (which causes botulism) from Clostridium tetani (which causes tetanus), for example.
However, the problem of being unable to identify bacteria at the genus level (a common problem with the equine gut microbiota) is like being unable to classify dogs even as members of the genus Canis. Rather, dogs would be classified simply as members of the family Canidae (the next level up, which includes the foxes), or at an even higher (and broader) level as carnivores (order Carnivora) or mammals (class Mammalia)—or simply as vertebrates (phylum, Chordata; subphylum Vertebrata), along with frogs and fish and french hens.
With few exceptions, studies of the equine faecal microbiota classify and analyse the relative abundance of bacteria at the genus level and the phylum level. But even at the phylum level, which is a huge ‘umbrella’ category four floors up from genus and five up from species level, equine faeces contain a disconcertingly high percentage of unidentified or unclassified bacteria. The more recent studies, using the more up-to-date databases, place the figure at somewhere between 5% and 12% of all OTUs. In other words, roughly 10% of OTUs found in the faeces of healthy horses cannot be classified even at the phylum level.
To be unable to identify or classify bacteria at the phylum level is somewhat like being unable to identify a horse as a vertebrate. The problem here is not that the test is too crude, like trying to identify a horse on a satellite image of Earth; rather, it’s that the microcosmos of the equine gut contains many species of bacteria that are, as yet, unknown to us or about which we still know too little.
Perhaps more than anything we do know about the microbiota of the equine gut, what we don’t know—the breadth and depth of our ignorance—is most striking. We'd be fools to meddle in a system we aren't even close to understanding in full. (How does that song go... "Fools rush in where angels fear to tread...")
Rethinking the 'core' microbiome
What all this means is that the well-worn concept of a 'core', common, shared, signature, or ‘equine’ microbiota may need to be revised. The following two studies illustrate why, because both show unequivocally that each individual has its own unique ‘core’ microbiota that is distinctly different from its herdmates.
The first study examined the faecal microbiota of three bands of semi-feral Welsh Mountain Ponies living free-range in the Carneddau mountains of northern Wales, UK. Each band consisted of one stallion and several mares, together with their juvenile offspring. In all, 30 ponies were studied.
- There are several great things about this study. One is the relatively large number of ponies included—most unusual for a study of the equine gut microbiota. Another is that these ponies were living a completely natural lifestyle and eating a completely natural diet, in the region where the breed originated.
The ponies were observed from a distance and fresh faecal samples collected on several occasions over a 3-month period from late summer to autumn (August to November). At least 3, and up to 5, faecal samples were collected from each pony over the course of the study.
The researchers looked at both the total faecal microbiome and the ‘core’ faecal microbiome, which they defined as gene sequence variants that were present in all individuals at a relative abundance of at least 0.001% of all faecal microbes.
They reported that the dominant bacterial Families represented in the core microbiome were "anaerobic bacteria associated with grass-eating mammals…" That makes sense; it’s also about as precise as we can reasonably be in trying to characterise the ‘core’ faecal microbiome of any group of horses. Here’s why:
Each pony had its own unique microbiome, but even that was hard to pin down because each pony’s microbiome varied by about 25% over the 3-month study period.
The plot illustrating the total faecal microbiome for each pony makes this point much better than I can with words:
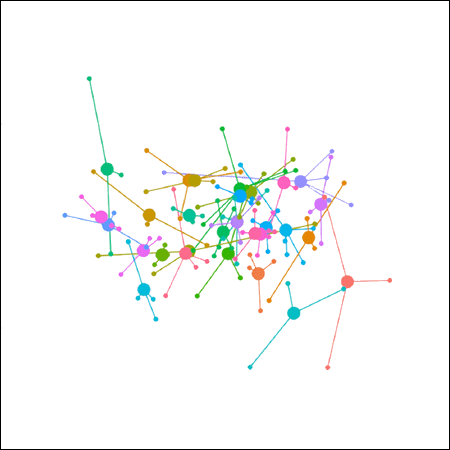
This plot represents the total faecal microbiome for the individual ponies. Each colour represents an individual pony (30 in total), and each small dot represents a separate faecal sample (3–5 per pony, 112 in total). Each large dot connecting the small dots of the same colour, like the hub of a wheel, represents the average or ‘centroid’ for that pony. The closer two points are, the more similar the microbial communities are for the two individuals or samples; the greater the distance between two points, the greater the difference between the two microbial communities.
This is essentially the plot that was published in the research paper. (I simply cleaned up the background and removed the X and Y axis markers, both of which extend from -1.0 to 1.0.) It looks like there’s a lot of overlap amongst the ponies, and with few exceptions a very similar microbiome—one might even say a ‘core’ microbiome. But look at how much variation there is among faecal samples from the same pony (distances between small dots of the same colour).
The large ‘centroid’ dots are tricking the eye because they’re not actual samples; they’re simply calculated “average” microbial communities for each pony. Take them away, along with the lines connecting the small and large dots, and see what happens...
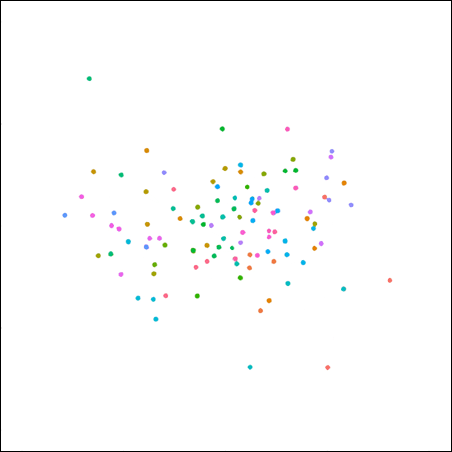
Plot of the same data, minus the artificial ‘centroid’ dots and connecting lines.
This plot is characterised more by its diversity—within and between ponies—than its similarity. This plot is the true representation of the study data.
As I mentioned, a great thing about this study is that these ponies were living a completely natural lifestyle and eating a completely natural diet that changed with the seasons. Except for a single annual roundup, these bands of essentially wild ponies were left to themselves, free to roam and graze whatever was available in their wilderness home. So, these data represent the faecal microbiomes of horses (ponies and horses are both Equus caballus) living ‘as nature intended’ for equidae.
We’ll come back to this study throughout the book because it’s such a treasure trove of data on the equine faecal microbiome, including the relationship between mare and foal, and broader social and geographic (dietary?) influences.
The important point here is that each horse’s gut microbiota is unique—and it is not entirely fixed (static), so it’s not entirely knowable. Even though we have the technology to ‘map’ an individual’s microbiome in great detail, the microbial community may vary considerably from one sample to the next even in a natural setting, so defining the ‘core’ microbiome even for an individual is rather slippery.
The next study bends the mind even further.
This second study involved dairy cows. There are some important similarities between the rumen in cattle and the hindgut in horses, enough so that this study is worth examining here.
Researchers took a pair of lactating dairy cows, both from the same herd and fed the same diet, and swapped their ruminal contents. In both cows, the solid and liquid contents of the rumen were scooped out through a surgically created fistula (a kind of porthole created to access the ruminal contents for study). Immediately after removal, the contents were transferred into the rumen of the other cow. The researchers estimated that they exchanged at least 95% of the ruminal contents from each cow, so it was practically a complete swap.
Prior to the study, the ruminal contents of several cows in the herd were sampled and mapped for their bacterial community composition. The two cows with the most dissimilar ruminal microbiota were selected for the study. The experiment was repeated several months later on a different farm, with two different cows, also selected because their microbial communities were most dissimilar among the group.
The point of the exercise was to see what happened to each cow’s ruminal microbiota over the next 2 months. As the researchers put it, “The purpose of this study was to examine the stability and host specificity of a cow’s ruminal bacterial community following massive challenge with ruminal microflora from another cow.”
We’ll come back to the ‘host’ (cow or horse) side of host specificity in Chapter 2 and microbial exchange from one animal to another in Chapter 3. Here I want to highlight what happened after ruminal exchange: in all of the four cows (two pairs) studied, the ruminal microbiota returned toward the cow’s original (pre-swap) bacterial community within 2 months. In one cow, it took only 2 weeks.
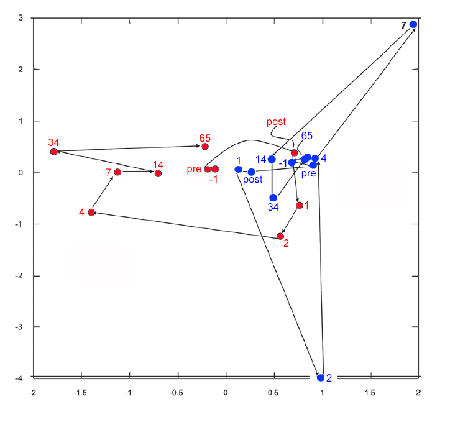
Plot of bacterial community composition in two dairy cows before and after near-total exchange of their ruminal contents. Each colour (red, blue) represents an individual cow. The text accompanying the coloured dots indicates the rumen sampling times, from the day before (-1), through the day of (pre and post), and up to 65 days after ruminal exchange.
In two cows, the ruminal communities 2 months after the swap were still substantially different than they were before the swap, but they were more similar to the individual’s original bacterial community than to that of the other (donor) cow.
In other words, the cows all made concerted efforts to restore their own, original ruminal microbial communities. Granted, this study involved only four cows, and cows are not horses. Even so, this study adds weight to the argument that each individual’s gut microbiota is unique—and it is conserved, even in the face of massive challenge.
Common themes
There are, however, some broad themes that are characteristic of healthy horses. Most of them happen at the phylum level.
The phylum Firmicutes predominates in the faeces of healthy horses, typically accounting for at least 50% and up to 80% of all faecal bacteria. Many of the bacteria in this phylum are fibre fermenters or 'fibrolytic', so it makes sense that they would predominate in an animal whose diet largely consists of plant fibre (at least, it should).
- -lytic or lysis means to disintegrate or break down, so fibrolytic bacteria break down dietary fibre
One of the best studies to date on the development of the hindgut microbiota in horses followed a group of 11 broodmares and their foals—all on the same farm and during the same foaling season—from foaling, through weaning (at around 6 months of age), until the foals were 9 months old. The phylum Firmicutes accounting for at least 60% of the faecal bacteria in all age groups after about 1 month of age.
Between 2 and 30 days of age, when foals are mostly consuming milk, Firmicutes dropped to only 40% of the total phyla, and the spectrum of faecal bacteria in this age group was quite unique. From 30 days of age onwards, when foals are eating increasing amounts of plant fibre, and when the normal foal behaviour of coprophagy (eating faeces) is at its peak, the spectrum of faecal bacteria became much more like that of the adult horses. (More on coprophagy and the development of the gut microbiota in foals in Chapter 3.)
This one study nicely illustrates both the development of the hindgut microbiota in normal foals and the influence of diet (particularly fibre intake) on the gut microbiota.
Some other themes common to healthy horses are worth noting. One is that the next-most abundant identifiable or classifiable phylum in the faeces or hindgut of healthy horses is either Verrucomicrobia or Bacteroidetes, depending on the study. You may never have even heard of Verrucomicrobia; and both it and Bacteroidetes comprise bacteria we know very little about in horses. There’s that pesky little problem (ignorance) again.
As important as it is to appreciate the tremendous number of different bacterial species (i.e., bacterial diversity or richness) in the faeces/hindgut, this approach doesn't tell us anything about the predominant types of bacteria in the hindgut—i.e., the bacteria that are present in greatest numbers and therefore are likely to have the greatest influence on digestion, gut function, and horse health.
For this reason, and because it's not always possible to clearly separate single OTUs from the 'background noise' in such crowded samples as faeces, the OTUs that occur with very low frequency are discounted in most studies of the equine gut microbiota. Instead, the relative abundance of the predominant bacteria at the phylum and genus level are examined. Yet here we are, able to identify the predominant phyla, but knowing little else about them.
As for the types of bacteria we are familiar with, Lactobacillus is not a predominant genus in the faeces or hindgut of healthy horses, and neither is Bifidobacterium (Bifidus) or Enterococcus. These bacterial genera are commonly used in probiotic products for humans and animals, including horses, yet they are not predominant bacterial genera in the equine hindgut.
In the mare-and-foal study, Lactobacillus accounted for only 1% to 2% of all bacteria in the faeces of mares and the foals older than 30 days, and at most only about 3% in young foals (whose diets were mainly milk). Bifidobacterium and Enterococcus didn't even rate a mention, as they were below the study’s threshold of 1% in all age groups.
On that farm, all mares and foals were turned out on pasture within 2 weeks of foaling. Their diets also consisted of grass hay, fed twice a day, supplemented with ‘sweet feed’ (grain-based concentrate, 12% crude protein) and beet pulp.
The importance of the horse’s diet on these three ‘probiotic’ genera is also illustrated in the study that examined the ‘core faecal bacterial microbiome’ in Irish Thoroughbred racehorses. In the four horses not in race training and being fed a forage-based diet (pasture or ‘haylage’), Lactobacillus accounted for, at most, 0.4% of the faecal bacteria. The proportion was higher in the two horses in race training and on high-starch diets; but even so, Lactobacillus accounted for only 0.8% of faecal bacteria in one horse and 2.6% in the other.
Also in that study, Bifidobacterium and Enterococcus each accounted for less than 0.2% of faecal bacteria, and were conspicuous by their absence in some horses. Bifidobacterium was not found in three of the four horses on forage-based diets, and it was present at extremely low levels (0.03% or less) in the two horses on high-starch diets. Enterococcus was found in four of the six horses, but at very low or extremely low levels (0.2% or less).
In another study, which compared the microbiota in the various segments of the gut in 11 horses, Lactobacillus predominated only in the stomach and duodenum (the first metre, 3 feet, or so of the small intestine). Whereas the relative abundance of this genus was about 48% in the stomach and duodenum, it had dropped to around 10% by the end of the small intestine (the ileum), and it was negligible in the caecum and the rest of the hindgut. So, any value of Lactobacillus as a probiotic genus in horses is likely to be confined to the stomach and small intestine; this is something we’ll explore further in Chapter 3.
However, in a study exclusively of the equine stomach, Lactobacillus species were the predominant bacteria in only two of the nine horses studied. In those two horses, Lactobacillus species had a relative abundance of, at most, 38%—and they comprised species that are unfamiliar to most of us: ‘sister group to L. jensenii and L. fornicalis’, L. hayakitensis, and L. equigenerosi. No L. acidophilus or other common probiotic species or strains; and no Bifidobacterium or Enterococcus.
In a comparative study of fecal microbiota in humans and farm animals (horses, cows, goats, rabbits, sheep, and pigs), Lactobacillus accounted for only 0.4%, Bifidobacterium 0.002%, and Enterococcus 0% of faecal bacteria in horses. One of the key study findings of interest to us here was that the microbiome of the equine gut was described as "notably different" from that of the human gut. That's not at all surprising—but it does suggest that probiotic products developed for humans may be a poor fit for horses.
Perhaps a good place to end this chapter is at the beginning (or near enough to it): the stomach. As we’ll see in Chapter 2, the stomach is a particularly hostile enviroment for bacteria, as it produces both hydrochloric acid and a protein-digesting enzyme (pepsin). Yet in a study examining the microbiota of the equine stomach, there were between 62 and 126 OTUs, or different bacterial species, in the stomach in the three horses kept in a stable and fed only hay. The average for this bland, boring diet was 78 OTUs per horse.
In contrast, the six horses kept at pasture and supplemented with hay—a much more varied and interesting diet—had between 80 and 226 OTUs (average, 128 OTUs) per horse. Statistical estimates put the upper limit at 166 OTUs for the hay-only diet and 287 OTUs for the pasture-based diet. In other words, this excoriating environment may be home to nearly 300 different bacterial species in grazing horses.
We’ll explore this distinction between a pasture-based diet and a hay-only diet further in Chapter 2, because it’s an important one that most studies don’t make. The point here is simply that, from a microbial perspective, the equine gastrointestinal tract is a veritable microcosmos: vast and richly populated, even on the ‘planet Mars’ of the equine gastric mucosa.
Bibliography
[In order of appearance]
Salter RE and Hudson RJ (1979). Feeding ecology of feral horses in western Alberta. Journal of Range Management 32: 221–225.
Abella SR (2008). A systematic review of wild burro grazing effects on Mojave Desert vegetation, USA. Environmental Management 41: 809–819.
Costa MC, Arroyo LG, Allen-Vercoe E, et al. (2012a). Comparison of the fecal microbiota of healthy horses and horses with colitis by high throughput sequencing of the V3-V5 region of the 16S rRNA gene. PLoS [Public Library of Science] ONE 7: e41484.
O'Donnell MM, Harris HMB, Jeffery IB, et al. (2013). The core faecal bacterial microbiome of Irish Thoroughbred racehorses. Letters in Applied Microbiology 57: 492–501.
Fernandes KA, Kittelmann S, Rogers CW, et al. (2014). Faecal microbiota of forage-fed horses in New Zealand and the population dynamics of microbial communities following dietary change. PLoS [Public Library of Science] ONE 9: e112846.
Sisson S (1975). Equine digestive system. In: Sisson and Grossman's The Anatomy of the Domestic Animals, 5th edition, volume 1, Getty R (editor). W.B. Saunders Co., Philadelphia, PA; pages 477–488.
Smyth GB (1988). Effects of age, sex, and post mortem interval on intestinal lengths of horses during development. Equine Veterinary Journal 20: 104–108.
Pfeiffer CJ, MacPherson BR (1990). Anatomy of the gastro-intestinal tract and peritoneal cavity. In: The Equine Acute Abdomen, White NA II (editor). Lea & Febiger, Malvern, PA; pages 2–24.
Lawrence LM, Cymbaluk NF, Freeman DW, et al. (2007). Energy Sources. In: Nutrient Requirements of Horses, 6th revised edition, Lawrence LM (chair), Committe on Nutrient Requirements of Horses, National Research Council of the National Academies. The National Academies Press, Washington, DC; page 6.
Mackie RI, Wilkins CA (1988). Enumeration of anaerobic bacterial microflora of the equine gastrointestinal tract. Applied and Environmental Microbiology 54: 2155–2160.
de Fombelle A, Jacotot E, Drogo ul C, et al. (1999). Effect of the hay:grain ratio on microbial and physico-chemical characteristics of cecal and colonic contents in ponies. Proceedings of the 16th Equine Nutrition and Physiology Symposium, Raleigh, NC; 16: 153–154.
de Fombelle A, Varloud M, Goachet A , et al. (2003). Characterization of the microbial and biochemical profile of the different segments of the digestive tract in horses given two distinct diets. Animal Science 77: 293–304.
Milinovich GJ, Burrell PC, Pollitt CC, et al. (2008). Microbial ecology of the equine hindgut during oligofructose-induced laminitis. The ISME [International Society for Microbial Ecology] Journal 2: 1089–1100.
Respondek F, Goachet AG, Julliand V (2008). Effects of dietary short-chain fructooligosaccharides on the intestinal microflora of horses subjected to a sudden change in diet. Journal of Animal Science 86: 316–323.
Muhonen S, Julliand V, Lindberg JE, et al. (2009). Effects on the equine colon ecosystem of grass silage and haylage diets after an abrupt change from hay. Journal of Animal Science 87: 2291–2298.
Lopes MA, White NA II, Crisman MV, et al. (2004). Effects of feeding large amounts of grain on colonic contents and feces in horses. American Journal of Veterinary Research 65: 687–694.
Costa MC, Silva G, Ramos RV, et al. (2015b). Characterization and comparison of the bacterial microbiota in different gastrointestinal tract compartments in horses. The Veterinary Journal 205: 74–80.
Furet JP, Firmesse O, Gourmelon M, et al. (2009). Comparative assessment of human and farm animal faecal microbiota using real-time quantitative PCR. FEMS [Federation of European Microbiological Societies] Microbiology Ecology 68: 351–362.
Williams S, Horner J, Orton M, et al. (2015). Water intake, faecal output and intestinal motility in horses moved from pasture to a stabled management regime with controlled exercise. Equine Veterinary Journal 47: 96–100.
Ingham ER (2001). Bacteria. In Soil Biology Primer [online] Tugel AJ, Lewandowski AM (editors). Last update: February 2001. https://extension.illinois.edu/soil/SoilBiology/bacteria.htm. Accessed on August 15, 2016.
Costa MC, Weese JS (2012b). The equine intestinal microbiome. Animal Health Research Reviews 13: 121–128.
Hart ML, Meyer A, Johnson PJ, et al. (2015). Comparative evaluation of DNA extraction methods from feces of multiple host species for downstream next-generation sequencing. PLoS [Public Library of Science] ONE 10: e0143334.
Shepherd ML, Swecker WS Jr, Jensen RV, et al. (2012). Characterization of the fecal bacteria communities of forage-fed horses by pyrosequencing of 16S rRNA V4 gene amplicons. FEMS [Federation of European Microbiological Societies] Microbiology Letters 326: 62–68.
Costa MC, Stämpfli HR, Allen-Vercoe E, et al. (2015a). Development of the faecal microbiota in foals. Equine Veterinary Journal 48: 681–688.
Proudman CJ, Hunter JO, Darby AC, et al. (2015). Characterisation of the faecal metabolome and microbiome of Thoroughbred racehorses. Equine Veterinary Journal 47: 580–586.
Zhao Y, Li B, Bai D, et al. (2016). Comparison of fecal microbiota of Mongolian and Thoroughbred horses by high-throughput sequencing of the V4 region of the 16S rRNA gene. Asian-Australasian Journal of Animal Sciences 29: 1345–1352.
Liu X, Fan H, Ding X, et al. (2014). Analysis of the gut microbiota by high-throughput sequencing of the V5–V6 regions of the 16S rRNA gene in donkey. Current Microbiology 68: 657–662.
Perkins GA, den Bakker HC, Burton AJ, et al. (2012). Equine stomachs harbor an abundant and diverse mucosal microbiota. Applied and Environmental Microbiology 78: 2522–2532.
Animal Microbiome Congress Europe 2020 web site. https://animalmicrobiomecongress.com/events/animal-microbiome-uk-2020. Accessed on January 8, 2020.
Schoster A, Arroyo LG, Staempfli HR, et al. (2013). Comparison of microbial populations in the small intestine, large intestine and feces of healthy horses using terminal restriction fragment length polymorphism. BMC [BioMed Central] Research Notes 6: 91.
Rodriguez C, Taminiau B, Brévers B, et al. (2015). Faecal microbiota characterisation of horses using 16 rdna barcoded pyrosequencing, and carriage rate of clostridium difficile at hospital admission. BMC [BioMed Central] Microbiology 15:181.
Antwis RE, Lea JMD, Unwin B, et al. (2018). Gut microbiome composition is associated with spatial structuring and social interactions in semi-feral Welsh Mountain ponies. Microbiome 6: 207.
Weimer PJ, Stevenson DM, Mantovani HC, et al. (2010). Host specificity of the ruminal bacterial community in the dairy cow following near-total exchange of ruminal contents. Journal of Dairy Science 93: 5902–5912.