Christine King BVSc, MANZCVS (equine), MVetClinStud
Precautions against ivermectin horse paste
to prevent or treat COVID-19 in people
- Please note: I am an equine veterinarian (horse doctor). I am neither licensed nor trained to treat humans, so I have taken pains in this article to avoid giving medical advice. That said, people I care about are choosing to take veterinary formulations of ivermectin for the prevention and early treatment of COVID-19. My friends, this article is written for you, in the spirit of helping to ensure that you do so with the least risk to your health.
As a follow-on to my article on ivermectin and the Hendra virus, I felt the need to write a commentary on people taking ivermectin paste for horses because they don't have access to the ivermectin formulation approved for human use.
The oral paste formulations of ivermectin approved for use as an anthelminthic (dewormer) in horses are a concern for a few reasons:
1. The horse paste is much more concentrated than the human formulation and the veterinary formulations licensed for use in smaller animals.
- Plain ivermectin horse pastes typically contain 18.7 mg of ivermectin per gram of paste (18.7 g/kg, or 1.87%), and a single tube is enough to treat a large horse (600- to 700-kg bodyweight, depending on the product).
- There is also a pelleted formulation of ivermectin for horses. It contains 140 mg of ivermectin per 35-gram sachet (= 4 mg of ivermectin per gram). One sachet is enough to treat a 700-kg horse. The manufacturer includes this caution: "not recommended for use in foals, due to risk of incorrect dosing and subsequent adverse outcomes. Avoid consumption of pellets by dogs."
- The ivermectin formulation approved for human use contains either 3 mg or 6 mg of ivermectin per tablet. Typical dosages for adults are in the range of 12 to 18 mg, depending on body weight.
- A widely available ivermectin formulation approved as a drench (oral liquid dewormer) for sheep contains 0.8 mg of ivermectin per ml (0.8 g/l, or 0.08%).
- 1 kilogram (kg) = 1,000 grams; and 1 kg = 2.2 pounds (lb)
- 1 gram (g) = 1,000 milligrams
- 1 milligram (mg) = 1,000 micrograms (mcg or µg)
- 1 litre (l) = 1,000 millilitres (ml) or 1,000 cubic centimetres (cc)
If it's this easy to get the dose wrong in horses, how much easier it would be to accidentally overdose or underdose a person with these horse products. And when it comes to drug safety and effectiveness, dose matters.
2. Ivermectin is often combined with other drugs for more broad-spectrum anthelminthic treatment. Unless you read the fine print on the box or tube, you may be getting more than just ivermectin in that tube of horse wormer.
- Note: this is not the same as the claims that these products may be "contaminated." They are not. Licensed products for veterinary use undergo strict manufacturing processes and quality controls similar to those required for human products. Think of it this way: if you're a manufacturer and your product is likely to be used on million-dollar racehorses, you'd be pretty darn careful!
- This concern is not about contamination or adulteration, but about other anthelminthic drugs (oxfendazole, oxibendazole, praziquantel, morantel, pyrantel, etc.) that may be included in that product for a legitimate purpose in horse management, such as the targeting of parasites that are not susceptible to ivermectin. Some of these drugs may be safe in humans (when taken in the right dosage for humans), but they have no known antiviral effects, and possible drug interactions in humans are unknown. In short, you do not want these other drugs in your system.
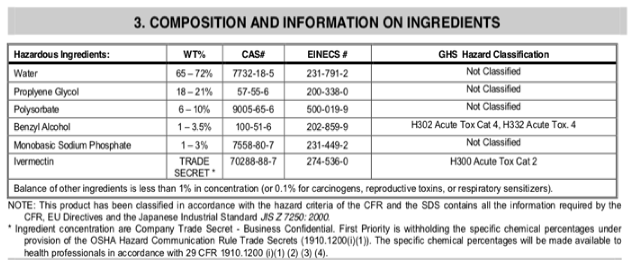
For example, one widely available ivermectin sheep drench in the US contains the following ingredients, in addition to ivermectin 0.08% (0.8 mg/ml, which is not a "trade secret," it's written on the label!):
- Actually, the TRADE SECRET is the specific proportions of avermectins that comprise the ivermectin component. According to Wiki, fermentation of the soil microbe Streptomyces avermitilis yields eight closely related avermectin forms; the majority are B1a and B1b. In a separate chemical step, the mixture is hydrogenated to produce ivermectin, which is an approx. 80:20 mixture of the two 22,23-dihydroavermectin compounds (B1a and B1b). This is the "trade secret" bit.
I've been unable to find comparable data for the ivermectin sheep drench in Australia, but it is likely to be very similar in composition.
Safety data
Ivermectin is used as an anthelminthic drug in a wide variety of animal species. It has a fairly good safety profile in most animals, although there are significant species differences and some very important breed differences, particularly in dogs. For example, the safety margin ranges from around 4x in dogs (but less than 1x in certain breeds) to around 50x in pigs. In horses, a single oral dose that is 10x the standard dose (0.2 mg/kg, or 200 µg/kg) is tolerated, but repeat that dose the next day and neurologic signs are likely to occur.
Ivermectin pastes for horses are safe for use in horses at the label dosages, but their use should be restricted to horses and other domestic equids (donkeys, mules). I would be hesitant to use the horse paste even in another livestock species (e.g., sheep or goats).
It is far safer to use a product that is formulated for the target species or that is known to be safe through experience (e.g., sheep drenches can safely be used in goats) and an understanding of the species similarities and differences in drug metabolism (e.g., dosages of sheep drenches in goats are different from those used in sheep).
human studies
In 2002, Merck (the company that developed ivermectin and first brought it to market) published a human study on ivermectin dosage in the Journal of Clinical Pharmacology. They concluded that ...
- "Ivermectin was generally well tolerated, with no indication of associated CNS [central nervous system] toxicity for doses up to 10 times the highest FDA-approved dose of 200 micrograms/kg."
So, the study indicated a safety profile in humans similar to that seen in horses.
The goal of that particular study was to investigate higher doses of ivermectin and more frequent dosing intervals than are currently approved for human use. Specifically, they examined single doses of 90 mg and 120 mg, as well as repeated doses of 30 mg and 60 mg given twice a week. This was a double-blind, placebo-controlled, dose escalation study. Here are some of the relevant highlights:
- "Adverse experiences were similar between ivermectin and placebo and did not increase with dose."
- The elimination half-life (the time it takes for 50% of the drug to be eliminated from the body) was approximately 18 hours. The authors concluded that "the accumulation of ivermectin given every fourth day is minimal."
- "This study demonstrated that ivermectin is generally well tolerated at these higher doses and more frequent regimens."
HOWEVER, a mini-review of ivermectin pharmacokinetics (how a drug moves through a body) in humans raises some little red flags for me, in relation to the use of formulations not intended for humans. This review was published in 2008, in the AAPS Journal (American Association of Pharmaceutical Scientists), by a group of pharmacologists in Spain. (The funding source is not specified, but I believe this was an independent review.)
Here are my main points of concern:
1. The peak plasma concentration (Cmax) varied with the formulation. It was about twice as high for the solution compared with the powdered formulations (tablet or capsule).
- Peak plasma concentration is the highest concentration of ivermectin achieved in an individual's bloodstream — the 'peak' — measured in nanograms (ng) of ivermectin per ml of plasma. As toxicity is related to plasma concentration, Cmax is important when discussing the safety of ivermectin. In this review of nine human studies, the Cmax ranged from about 20 ng/ml to 80 ng/ml, depending on dose and formulation (solution or tablet/capsule).
- In Australia, the package insert states the following: "Ivermectin is incompletely absorbed (~50% bioavailable relative to an oral hydroalcoholic solution) following oral doses of ivermectin tablets, with a Tmax of ~4 hours. With 12 mg single dose tablets administered in healthy male volunteers, the mean peak plasma concentration of the major component was 46.6 (± 21.9) ng/mL (range 16.4-101.1 ng/mL)."
The oral ivermectin product licensed for use in humans is a tablet. The sheep drench is a solution. The horse paste is a bit of a mystery, because we don't know its base ingredients, but it is probably somewhere in between the two and more 'wet' than 'dry'.
So, ivermectin toxicity may be more likely with the sheep drench and horse paste simply because of the different absorption characteristics between dry and liquid formulations of ivermectin in humans.
If you have chosen to take a liquid or paste formulation of ivermectin, it may be safest to use the lower end of the dose range advised by the doctors who are prescribing or recommending the human formulation of ivermectin for the prevention and treatment of COVID-19.
2. The elimination half-life (t1/2), which the authors described as being “around a day,” ranged from 11 hrs to almost 55 hrs. In most studies it was in the range of 12–24 hrs, but in one study it was around 36 hrs, and in another it was 54.5 hrs.
- As a reminder, t1/2 is the time it takes for 50% of the drug to be eliminated from the body. In the Merck study I mentioned earlier, the t1/2 of oral ivermectin in humans was approx. 18 hours, which is consistent with this review.
- Note: t1/2 doesn't change with the dose, because half-life is a measure of how long it takes for 50% of the absorbed dose to be eliminated, no matter how high or low the plasma concentration was at its peak. That said, the higher the dose, the longer it takes for the drug to drop below effective levels in the bloodstream and tissues.
These figures are for a single oral dose. They don't tell us what happens when a person takes a second, third, fourth, or fifth dose. Here is where t1/2 becomes very important. The trick is to maintain effective levels of the drug in the bloodstream and tissues, without reaching toxic levels. Repeat the dose within the t1/2 window, and the drug can accumulate in the body (bioaccumulate), potentially reaching toxic levels after multiple doses.
- While the t1/2 for oral ivermectin in humans was "around a day" (24 hours) in most studies, it was quite a bit longer in a couple of studies. What these studies tell us is that repeated daily dosing will result in bioaccumulation of ivermectin that in some people could reach potentially toxic levels.
- In the Merck study, ivermectin doses of 30 mg twice a week (on days 1, 4, and 7) resulted in total plasma concentrations over time ('area under the curve') that were 1.24 times (24%) higher on day 7 than on day 1. Ivermectin doses of 60 mg twice a week resulted in total plasma concentrations over time that were 1.4 times (40%) higher on day 7 than on day 1. They took these results to mean that bioaccumulation is minimal with this dosing regimen. While that is a fair statement in the context of this study, these data also show that ivermectin bioaccumulates even when the doses are spaced 3 days apart.
The most recent ivermectin protocols I’ve seen for the prevention and early outpatient treatment of COVID-19 recommend repeated doses of ivermectin at variable intervals from daily to twice a week, depending on the circumstances. Given the pharmacokinetics of ivermectin in humans, the drug could quickly accumulate with daily dosing, particularly if taking a more bioavailable formulation such as horse wormer or sheep drench.
These protocols typically cap the daily dosing of ivermectin at 5 days in patients who are self-medicating (for prevention or early outpatient treatment). Pay attention to the dosing recommendations by experienced doctors who are using ivermectin in their hospitals and clinics. This drug does have a fairly good safety profile in humans, but overdose is certainly possible with daily dosing.
If you have chosen to take a liquid or paste formulation of ivermectin, it may be safest to use a longer interval between doses than the one(s) advised by the doctors who are prescribing or recommending the human formulation of ivermectin for the prevention and treatment of COVID-19.
3. There may be significant gender differences in the clearance of ivermectin from the body. In one study of healthy people given 150 µg/kg (0.15 mg/kg) of ivermectin, total body clearance of ivermectin was almost twice as high in women as in men.
So, if clearance is lower in men (a big IF, as that was just one study), then repeated daily dosing may be more likely to cause toxicity in men than in women.
If you have chosen to take a liquid or paste formulation of ivermectin and you are male, it may be safest to use a longer interval between doses than the one(s) advised by the doctors who are using the human formulation of ivermectin for the prevention and treatment of COVID-19.
4. Ivermectin is a highly lipophilic molecule, meaning that it has a high affinity for fats (lipids). Of the body tissues examined in ivermectin distribution studies, “Fat showed the highest and most persistent levels..."
- Its lipophilic nature probably explains the fairly long elimination half-life (12–24 hrs) for oral ivermectin in people. The t1/2 is even longer in horses with the oral paste formulation (4–5 days in one study).
- That's yet another reason to avoid taking ivermectin paste formulated for anthelminthic use in horses. We don't know what else is in it that might delay the absorption and/or elimination of the ivermectin component. In the study referenced above, the Cmax in horses given ivermectin paste at a dose rate of 0.2 mg/kg was comparable to that reported in people (average 22 ng/ml, range 8–40 ng/ml). However, the time to peak plasma concentration (tmax) was quite a bit longer: average 10 hrs (range 2–24 hrs) in the spring when the horses were on pasture, and 22 hrs (range 2–36 hrs) in the autumn when the horses were mostly fed hay. Imagine that: in some horses it took 36 hrs for them to reach Cmax after a single oral dose of ivermectin paste!
- In the mini-review of ivermectin pharmacokinetics in humans, the tmax for all formulations of ivermectin averaged around 5 hrs (range 3.4–10.3 hrs). So, there may well be components in the ivermectin horse pastes that are designed to delay absorption and thus extend the elimination half-life of ivermectin for anthelminthic effect. However, this is just a guess. As I've said, we don't know what else is in these paste formulations for horses.
So, because ivermectin is such a fat-loving molecule, significant accumulation of ivermectin with repeated dosing may be more likely in people with greater amounts of body fat than in lean people.
If you have chosen to take a liquid or paste formulation of ivermectin and you are overweight, it may be safest to use a longer interval between doses than the one(s) advised by the doctors who are using the human formulation of ivermectin for the prevention and treatment of COVID-19.
The sad irony here is that being overweight is a specific risk factor for COVID-19. Please, if you are ... er ... 'generously padded', take good care of yourself, and go easy with the ivermectin.
5. Food and drink affects ivermectin absorption, but not in consistent ways.
(a) Alcohol may increase the absorption of ivermectin.
- In a group of healthy volunteers given 150 µg/kg of ivermectin with either beer or water, the plasma concentration at various time points from 1 to 4 hours after a dose was 150% to 165% higher when ivermectin was consumed with beer than with water.
(b) Fruit juice may decrease the absorption of ivermectin.
- In a group of healthy volunteers given 150 µg/kg of ivermectin with either orange juice or water, the peak plasma concentration (Cmax) was 39% lower when ivermectin was consumed with OJ than with water. Similarly, the total plasma concentration over time (area under the curve) was 35% lower with OJ than with water.
- The authors of the review speculated that the reason may be because fruit juices are potent inhbitors of certain drug transporters. Grapefruit juice is perhaps best known for this effect, but other fruit juices can have similar effects on drug absorption or metabolism.
(c) A fatty meal may increase the absorption of ivermectin.
- In one part of the Merck study, healthy volunteers were given 30 mg (333 to 600 µg/kg) of ivermectin after an overnight fast and either on an empty stomach (no food for 4 hours after the dose) with just a glass of water, or straight after a fatty breakfast. The fatty meal contained 31 grams of protein, 57 grams of carbohydrates, and 49 grams of fat, for a total of 784 kcal (3,280 kJ).
- The peak plasma concentration (Cmax) of ivermectin was 3 times higher after a fatty meal than with a glass of water on an empty stomach. The area under the curve was 2.6 times higher after a fatty meal than with a glass of water on an empty stomach.
- Note: in Australia, the package insert for humans advises taking ivermectin tablets with a full glass of water. No mention is made of food.
If you have chosen to take a liquid or paste formulation of ivermectin, avoid taking it with alcohol, fruit juice, or a fatty meal. What is already a gamble becomes a bigger risk when these other influences are in play.
6. The pharmacokinetics of ivermectin have not been studied in people with impaired liver or kidney function, nor in people aged 65 years and older.
As ivermectin is primarily metabolised by the liver and eliminated in the faeces (stool, 'poop'), patients with impaired liver function should probably be especially careful with this drug.
Merck issues this warning in the package insert for their Australian product: "Clinical studies of [their ivermectin tablets] did not include sufficient numbers of elderly subjects aged 65 years and over to determine whether they respond differently from younger subjects. In general, treatment of elderly patients should be cautious, reflecting the greater frequency of decreased hepatic [liver], renal [kidney] or cardiac [heart] function, and of concomitant disease or other drug therapy.
- "Very rare post-marketing reports of increased INR (International Normalised Ratio) have been reported when ivermectin was co-administered with warfarin."
- "In clinical trials involving 109 patients given either one or two doses of 170-200 µg/kg ivermectin, the following laboratory abnormalities were seen irrespective of drug relationship: elevation in ALT and/or AST (2%), decrease in leukocyte count (3%). Leukopenia and anaemia were seen in one patient."
If you have chosen to take a liquid or paste formulation of ivermectin and your liver is impaired or if you are over 65 years of age, it may be safest to use a lower dose and a longer interval between doses than the one(s) advised by the doctors who are using the human formulation of ivermectin for the prevention and treatment of COVID-19.
We still don't know enough about this drug as an antiviral agent, and optimal dosage (dose and dosing interval) is one important aspect that we are still exploring. In the meantime, the precautionary principle seems prudent: proceed with caution or don't do it at all if there is significant potential for harm.
© Christine M. King, 2021. All rights reserved.
First published 01 September 2021. Last updated 08 September 2021.