
animal health consulting
Ivermectin and the Hendra virus
lessons from COVID-19
Christine King BVSc, MANZCVS (equine), MVetClinStud
The COVID-19 pandemic has resulted in some surprising discoveries. One is that ivermectin (yes, that ivermectin, the anthelminthic drug) is a broad-spectrum antiviral agent, inhibiting a wide variety of viruses.
Ivermectin is antiviral
It's been known for awhile in research circles (e.g., in virology labs) that ivermectin has antiviral properties. However, it hasn't been used clinically (i.e., in patients with naturally occurring disease) as an antiviral agent until now.
Based on laboratory confirmation that ivermectin inhibits SARS-CoV-2 (the novel coronavirus that causes COVID-19), doctors in many different countries have been using ivermectin in the treatment and prevention of COVID-19. More on that in a bit.
It turns out that ivermectin inhibits a wide variety of viruses, including Hendra virus (HeV). Here is a list of some of the viruses known to be inhibited by ivermectin, at least in the laboratory:
* Adenoviruses (among the causes of the common cold)
* Coronaviruses (CoV), notably SARS-CoV-2
* Dengue viruses
* Equine herpesvirus type 1 (EHV-1, isolated from an aborted equine foetus)
* Hendra virus (HeV)
* Human immunodeficiency virus (HIV)
* Influenza viruses (in particular, avian influenza A, or 'bird flu')
* Pseudorabies virus, or suid herpesvirus type 1 (causes Aujeszky's disease or 'mad itch' in various animals, including dogs, cats, and farm animals)
* Venezuelan equine encephalitis (VEE) virus
* West Nile virus (WNV)
* Yellow fever virus
* Zika virus
This list covers several different viral families, making ivermectin a broad-spectrum antiviral agent.
A recent laboratory study showed that ivermectin also inhibits the viruses that contribute to the bovine respiratory disease (BRD) complex in cattle:
* Bovine coronavirus (BoCV)
* Bovine parainfluenza virus type 3 (BPIV-3)
* Bovine viral diarrhoea virus (BVDV)
* Bovine respiratory syncytial virus (BRSV)
* Bovine herpesvirus type 1 (BoHV-1)
The authors of the cattle study stated, "Consequently, IVM [ivermectin], which is licensed for antiparasitic indications, also deserves to be evaluated as a broad-spectrum antiviral in BRD cases caused by viral pathogens."
How it works
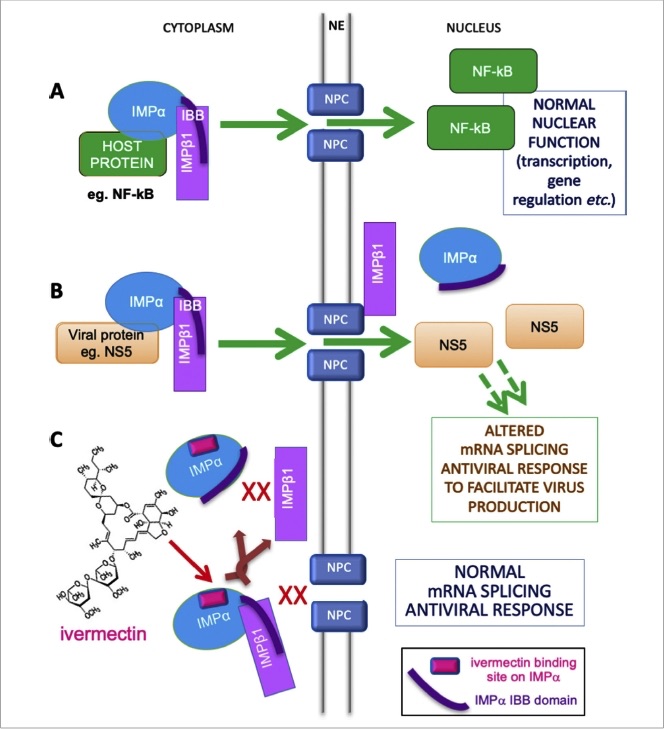
The way ivermectin inhibits viral activity within a cell is complex — because viral replication is a complex process. In essence, ivermectin blocks the virus from entering the cell's nucleus and, once there, disabling a key component of the cell's antiviral response. In other words, ivermectin blocks one of the critical steps involved in viral replication.
A note of caution before we go on: the molecule blocked by ivermectin — called importin alpha, or IMP𝛂 — is a nuclear transporter protein that aids the transport ('import') of molecules from the cytoplasm of the cell into the nucleus. However, IMP𝛂 is involved in a variety of normal cell functions, including antiviral responses.
So, although many different viruses 'hijack' this transporter protein for their own purposes, it is not a good idea to persistently block IMP𝛂 in order to block viral replication. To do so would block normal cell functions as well — including the ability to mount an antiviral response!
Here is where dosage is key — not just the amount of ivermectin given, but how often the dose is repeated (how many doses, at what interval between doses, and for how long).
Dosage
We don't yet know the optimal dosage of ivermectin as an antiviral agent. Its clinical use in this regard is still very new, and much is yet to be learned. However, we can begin to make some tentative deductions.
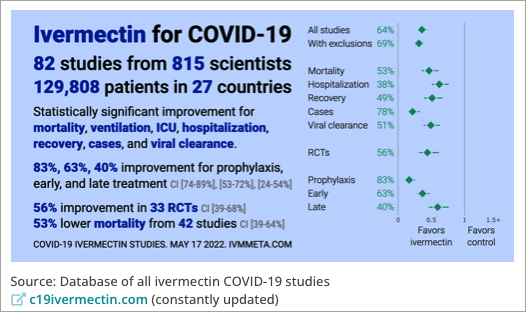
By January 2021, there were almost 70 human clinical trials registered and underway worldwide on the safety and effectiveness of ivermectin for the treatment or prevention of COVID-19 in humans, and there have been many more since. At the present time (18 May 2022), 82 human clinical trials have been completed, involving over 800 scientists in 27 countries, and close to 130,000 patients.
Model of IMPɑ′s role in nuclear transport of host and viral proteins, and mechanism of inhibition by ivermectin. A. Normal function. B. Viral hijacking. C. Inhibition by ivermectin.
Abbreviations: IBB, IMP𝛃-binding region of IMP𝛂; IMP𝛂, importin alpha; IMP𝛃1, importin beta-1; NE, nuclear envelope; NF𝜿B, nuclear factor kappa B; NPC, nuclear pore complex, NS5, a specific viral protein.
Note: Not all viruses use or rely exclusively on IMP𝛂, so ivermectin is not effective against all viruses, just against those that rely on IMP𝛂 for their replication, such as those listed above.
The striking thing to me is that many of those trials used ivermectin dosages that are the same as we use in horses as an anthelminthic agent (dewormer): 0.2 mg/kg (200 µg/kg) bodyweight. This dosage is also used for anthelminthic treatment in humans, which is probably why it was chosen in numerous clinical trials: it is known to be well tolerated in humans.
µg is the symbol for microgram; it may also be written as mcg (not to be confused with mg...)
1 gram (g) = 1,000 milligrams (mg)
1 milligram (mg) = 1,000 micrograms (µg or mcg)
1 microgram (µg) = 1,000 nanograms (ng)
Higher dosages (up to 0.6 mg/kg, or 600 µg/kg, bodyweight) are used in humans for certain parasitic diseases, and these higher dosages have been used in some of the COVID-19 trials and in at least one current treatment protocol (see below).
Some trials used a single oral dose of ivermectin; others repeated the dose daily for up to 7 days, or gave 2 doses spaced at least 2 days apart (e.g., on Days 1 and 3). The optimal dosing regimen remains to be determined, but in none of these trials was dosing repeated for more than 7 days in a row.
A comprehensive review was published in January 2021 by the Front Line COVID-19 Critical Care Alliance (FLCCC), a group of critical-care physicians who use and recommend ivermectin in their COVID-19 treatment and prevention protocols.
A more recent systematic review and meta-analysis by another group was published in the American Journal of Therapeutics in June 2021. It further confirmed the clinical value of ivermectin in COVID-19 at these commonly used dosages (o.2–0.6 mg/kg bodyweight).
In September 2021, the FLCCC addressed the safety of ivermectin, including 'high-dose' ivermectin (0.6 mg/kg, or 600 µg/kg), linking to the relevant studies which show that ivermectin is well tolerated at 3x the standard dosage (which is 0.2 mg/kg, or 200 µg/kg ). The FLCCC keeps current on publications related to the use of ivermectin in COVID-19, so please check with them for the latest findings and recommendations.
The current FLCCC guidelines, published in January 2022 (version 19) and revised with the delta and omicron variants of SARS-CoV-2 in mind, advise the following:
Prevention of COVID-19
Post-exposure, ivermectin: 0.4 mg/kg once; repeat in 48 hours
For use if a household member tests positive for COVID-19 or you've otherwise had prolonged exposure to a person who is positive for COVID-19 while not wearing a mask.
Ongoing prevention, ivermectin: 0.2 mg/kg twice a week (i.e., once every 3–4 days), for as long as disease risk is elevated in your community
Note: This preventive protocol also includes supplemental vitamin D3, vitamin C, quercetin, melatonin, zinc, and a gargled antiseptic mouthwash. It does not rely on ivermectin alone.
Early treatment of COVID-19
Ivermectin: 0.4–0.6 mg/kg daily for 5 days (or until recovered)
Use the higher dosage (0.6 mg/kg) in these circustances: (1) in areas where aggressive variants (e.g., delta) are circulating; (2) when treatment is started on/after day 5 of symptoms or in the pulmonary (breathing difficulty) phase of COVID-19; or (3) you have multiple co-morbidities (other chronic disease/s) or risk factors.
Note: This early treatment protocol also includes supplemental vitamin D3, vitamin C, quercetin, melatonin, zinc, and aspirin, along with oral/nasal 'sanitation' treatments. It does not rely on ivermectin alone.
This higher ivermectin dosage is also used to treat hospitalised patients, along with various other medical interventions as needed.
Long-haul COVID and adverse vaccine effects
Interestingly, ivermectin is included in the FLCCC's guidelines for treating long-haul COVID syndrome and for post-vaccine inflammatory syndromes associated with COVID-19 vaccination. The ivermectin dosage advised in these situations is 0.2 mg/kg once a day for 7 days, along with the other measures recommended for these conditions.
What does all this mean for viral infections in horses?
This research makes my whiskers twitch, and it raises a lot of questions for me.
An obvious one is whether ivermectin might be useful in the treatment of serious viral infections in horses for which we don't currently have very good or cost-effective treatments. In Australia, they include the neurologic form of equine herpesvirus type 1 (EHV-1), Murray Valley encephalitis virus, Kunjin virus (Australian strain of West Nile virus), and, of course, Hendra virus (HeV). It is possible that horses with Ross River virus infection might also benefit, although I have not yet found definitive evidence that ivermectin blocks this particular virus.
If nothing else, ivermectin is anti-inflammatory by virtue of being a potent inhibitor of NFkB.
Given the potential for HeV to spread to other horses and to humans, it is reasonable to ask whether we should even be attempting to treat HeV infection in horses. I would answer that with an emphatic yes! It's not the politically correct answer, but it's a medically sound one — not to mention a compassionate one.
As I discuss in my multi-part article on HeV in horses, HeV infection is not universally fatal in horses (nor in humans); some infected horses recover — and others don't even become ill when infected with HeV. Furthermore, experimental studies have shown that HeV is not highly contagious.
In the first experiment of a 3-part disease transmission study using flying-foxes, horses, and cats, 8 flying-foxes were inoculated with HeV and housed in contact with 3 uninfected flying-foxes and 2 uninfected horses.
None of the flying-foxes or horses became ill, although 6 of 8 inoculated bats developed antibodies against HeV, and 2 of 6 had vascular (blood vessel) lesions on postmortem examination which contained viral antigen. The in-contact bats and horses did not develop HeV antibodies, indicating that they had not been infected with HeV.
In the second experiment, 4 horses were inoculated with HeV by subcutaneous injection and intranasal inoculation, and housed in contact with 3 uninfected horses and 6 uninfected cats.
Three inoculated horses became ill, but the fourth horse did not, despite being inoculated with HeV. The in-contact horses and cats did not become infected.
In a third experiment, 12 cats were inoculated with HeV and housed in contact with 3 uninfected horses. One horse became ill, but the other 2 horses did not.
In this study, transmission from flying-foxes to horses could not be proven and neither could transmission from horses to horses or horses to cats. The authors concluded, "Under the experimental conditions of the study [which were designed to encourage viral transmission], HeV is not highly contagious."
A similarly low rate of person-to-person transmission is reported for the closely related Nipah virus (NiV) in people, who can be directly infected with NiV from contact with bat excretions. In one study, only 35 of the 2,494 people (1.4%) who came in contact with a NiV patient probably acquired NiV through person-to-person transmission. Spouses of NiV patients were infected more often (14%) than other close family members (1.3%) or unrelated contacts (0.9%).
Yes, let's isolate any suspect case while awaiting HeV test results. And let's take other appropriate precautions, such as using personal protective equipment, to ensure that we don't become infected ourselves or spread the virus to other animals. But let's also treat these sick horses while we're waiting!
Where's the harm in giving a single oral dose of ivermectin — properly suited up, of course — while we wait on the HeV test results?
And even if the test comes back positive for HeV, where's the harm in continuing to treat the horse symptomatically and with ivermectin, guided by what we've learned from the human clinical trials of ivermectin for the treatment and prevention of COVID-19?
This approach could also be extended to exposed but still-healthy horses on the same or neighbouring farm, and even to the humans handling the sick horse.
As to that, a monoclonal antibody-based HeV treatment for humans cleared the first hurdle — safety in healthy human volunteers — in early 2020. The next step is to complete a clinical trial that documents its effectiveness for the treatment and prevention of HeV infection in humans exposed to HeV. "The antibody has been available in Queensland since 2010 for the treatment of HeV infection in people. Since then, it has been used on compassionate grounds in 13 people in Queensland."
A note of caution about using ivermectin prophylactically in horses (i.e., to prevent HeV infection):
Although it may turn out to be a worthwhile preventive strategy in still-healthy horses exposed to a HeV-infected horse, it is not a wise long-term strategy.
Quite apart from the fact that ivermectin blocks a normal and necessary nuclear transporter protein, ivermectin is still one of the most important anthelminthic drugs we have for horses. Overuse speeds the development of drug resistance in parasites, not just to ivermectin but to all other drugs in its class (abamectin, moxidectin, etc.).
If you are choosing not to vaccinate your horse against HeV, then save the ivermectin for situations in which there is a fairly high degree of risk that your horse has been exposed to HeV, whether from a sick horse or directly from flying-foxes. And then use it only for the period of high risk.
Because we need ivermectin to remain a highly effective anthelminthic drug in horses, it would be better to resolve the risk of HeV infection so that ongoing treatment with ivermectin is unnecessary. Rather than continuing with ivermectin twice a week, for example, as is currently recommended for COVID-19 in high-risk people, do whatever is needed in the situation to prevent further exposure to HeV.
Dosage
As for the dosage of ivermectin as an antiviral agent in horses, here is another important unknown.
Pharmokinetics is the science of how a drug moves through the body. It includes absorption, distribution, retention, and elimination characteristics, and thus it determines the optimal dosage of that drug in that species.
The pharmacokinetics of ivermectin oral paste in horses are substantially different from those of ivermectin tablets in humans.
With the standard ivermectin dosage of 0.2 mg/kg bodyweight, the maximum or 'peak' plasma concentration (Cmax) is very similar in horses and humans (20–80 ng/ml). In other words, the same oral dosage reaches about the same peak plasma concentration in both species, which is why the standard anthelminthic dosage of ivermectin is the same in both species. Distribution throughout the body is also similar for horses and humans, as is the main route of drug elimination (via the liver, into the bile and thus the faeces or stool).
So far, so good...
However, the time it takes to reach this peak (called tmax) is very different between horses and humans:
* in humans, the average tmax for oral ivermectin is ~ 5 hours (range, 3–10 hours); in other words, the dose you take at breakfast typically reaches its peak plasma concentration by lunchtime
* in horses, the average tmax for ivermectin oral paste is 2–4 times longer, is much more variable, and is dependent on the horse's diet
For example, in the referenced study, the tmax averaged 10 hours (range 2–24 hours) in the spring when the horses were on pasture, and a whopping 22 hours (range 2–36 hours) in the autumn when the horses were mostly fed hay
This species difference in tmax will affect the optimal dosing interval — but not nearly as much as the species differences in elimination half-life...
Elimination half-life (t1/2) is the time it takes for 50% of the absorbed dose to be eliminated from the body. It is a very important component of determining the dosage, especially the interval between doses when using a drug that will be given more than once. The t1/2 of oral ivermectin is very different between horses and humans:
* in humans, the average t1/2 for oral ivermectin is ~ 18 hours (12–24 hours, although across studies it has ranged from 11 to 54 hours); in other words, by this time tomorrow, the average person will have eliminated ~ 50% of the dose they just took
* in horses, the average t1/2 for ivermectin oral paste is 4–5 days. Days! Daaaaays!!
With any drug in any species, if you repeat the dose before t1/2, you risk increasing the plasma concentration of the drug beyond therapeutic or prophylactic (preventive) levels, and into the toxic range.
Ivermectin has a good safety profile in horses. It is considered safe up to about 10x the standard dosage. In other words, a single oral dose of 2 mg/kg (10 x 0.2 mg/kg) is tolerated in most adult horses. The same is true in humans.
However, ivermectin toxicity is well documented in both foals and adult horses. It manifests primarily as central nervous system disorder (unsteadiness [ataxia], severe depression, blindness, facial paralysis, hyper-reactivity, abnormal stance, etc.).
Repeated administration of ivermectin at an interval shorter than t1/2 (4–5 days in horses), particularly at dosages well above 0.2 mg/kg bodyweight, is more likely to cause toxicity.
The 'high-wire act' when using ivermectin as an antiviral agent in horses — in the absence of any equine-specific research or clinical experience — is to keep the plasma ivermectin concentration in the therapeutic–prophylactic range throughout the period of risk, without exceeding its threshold and entering the toxic range.
Based on what we know about the incubation period for HeV following natural exposure in horses (5–16 days), the pharmacokinetics of ivermectin oral paste in horses, and the safety profile of ivermectin in horses, the following approach seems prudent for sick horses with HeV or still-healthy but exposed horses at high risk of contracting HeV infection:
* single oral ivermectin dose of 0.4 mg/kg (i.e., 2x the standard anthelmintic dose, knowing that up to 10x the standard dose is tolerated in healthy horses)**
** Note: I would probably use the standard 0.2 mg/kg dosage in sick horses showing neurologic signs of viral infection. These horses likely have a compromised blood-brain barrier caused by viral invasion and the associated inflammatory response, so ivermectin toxicity may be more likely to occur (and to complicate matters) in these individuals.
But not all horses with HeV infection show neurologic signs; many show primary signs of respiratory disease or simply a general malaise (inapppetance, lethargy, fever, etc.). In the absence of neurologic signs, I would probably use the higher dosage (0.4 mg/kg) in the hope of achieving a greater antiviral effect.
Incidentally, signs of ivermectin toxicity are reversible. They resolve on their own in time (improvement in 12–24 hours, complete recovery within 4 days), and recovery can be accelerated with intravenous lipid therapy, and possibly with oral lipids (fats or oils).
* repeat in 5–7 days, at a dosage of 0.2–0.4 mg/kg for sick horses (as above) and 0.2 mg/kg for still-healthy but exposed horses
* then reassess; if risk or signs of illness persist, give a third dose 1 week later
This approach covers the 2+ weeks of uncertainty in exposed but still-healthy horses; and in sick horses with HeV it continues a (hopefully) therapeutic antiviral plasma concentration for up to 3 weeks, by which time they should be well on the road to recovery (or, to use the brutalist phrase, they're "dead or better").
***
As we learn more, I expect to have to revise these preliminary suggestions. This is simply the approach I plan to take with the next horse who has a serious illness that appears to be caused by a virus which, at least in the laboratory, is susceptible to ivermectin.
We must also keep in mind that the FLCCC guidelines do not rely on ivermectin alone in any of the clinical scenarios in which they advise its use: prevention, early (at-home or outpatient) treatment, late (in-hospital) treatment, long-haul or post-COVID syndrome, and post-vaccine inflammatory syndromes. Ivermectin is an important antiviral component in their various protocols, but it is not a stand-alone drug, and they do offer alternatives, both pharmaceutical and nutritional/herbal (e.g., Nigella sativa, or black cumin seed, as an alternative or adjunct to ivermectin).
There is still much for us to learn. But that should not stop us from at least trying to help horses with serious viral infections who might otherwise die or suffer permanent damage were we to continue to limp along with only the currently accepted treatments. With due care, adding ivermectin to the treatment regimen for these horses would surely fall into the "can't hurt, might help" basket — and I can think of at least one patient I lost who might be alive today had I known all of this sooner.
© Christine M. King, 2020, 2022. All rights reserved.
First published 04 July 2021.
Last updated 18 May 2022.
Screenshot from Frontline COVID-19 Critical Care Alliance (FLCCC) website, 18 May 2022.